It is more than 60 years since the first evidence emerged of the existence of stem cells and their natural ability to renew and differentiate into specialised cells types, such as those governing the blood, brain, heart or bones. Two Canadian scientists happened to observe colonies of proliferating blood cells after injecting bone marrow cells into mice. Given that the rodents’ blood cells were able to regenerate fully, exciting potential medical benefits for humans were suddenly envisaged.
Stem cells are considered the body’s master cells – the raw materials or primitive cells from which all other cells originate. Crucially, in the right conditions, be it in the body or a laboratory, stem cells can be split into daughter cells that are able to form new stem cells or, through differentiation, morph into specialised cells that have more specific functions.
Stem cell research offers an advanced method of studying the function of genes and physiological processes, yet the really interesting and far-reaching consequences of the original findings in 1961 are the vast range of possible clinical applications. As well as shedding light on the origins of cancer, stem cells can potentially drive therapies for the treatment of diabetes, spinal cord injury, heart disease, Alzheimer’s disease, Parkinson’s disease and more.
Government warning
Stem-cell therapies replace ailing patients’ damaged tissues using stem cells (or their daughter cells). Such treatments involve advanced therapy medicinal products (ATMPs) – medicines based on genes, tissues or cells – and are considered high- risk. In many countries, they fall under statutory regulations and citizens are advised to be wary of unproven cell-based therapies offered by some clinics around the world.
In Hong Kong, no ATPMs in the stem-cell category are registered for use. The Department of Health’s Drug Office warns that the safety, efficacy and quality of any unregistered medicines using stem-cell technology cannot be guaranteed. Certain cell or tissue replacement therapies like blood transfusion and bone marrow and cornea transplants, however, do not fall under this category and are commonly practised.
Dangerous hype
The potential to replace damaged tissue through regenerative medicine has fascinated the scientific community for decades. For laypeople who read about stem cells being coaxed into multiplying and populating different tissues, it may seem like a wonder cure for all debilitating conditions. This wild speculation has been fuelled by apparently successful cases being claimed and blown out of proportion before rigorous scientific process has been conducted.
A particularly notorious case involved Italian thoracic surgeon Paolo Macchiarini, who in 2008 created a new airway for Claudio Castillo, a young woman from Barcelona, using stem cells taken from her own bone marrow. These were implanted into a windpipe taken from a deceased donor, and stripped of its cells to leave a bare scaffold. Since the treatment involved the patient’s own cells, it appeared that her immune system was able to accept the replacement windpipe without the necessity of immune-suppressing drugs. The organ seemed to function like it was Castillo’s own, and the procedure was reported in the press as a miraculous breakthrough.
But this lofty claim was not justified. Many of the 17 or more patients around the world whom Macchiarini treated with artificial or regenerating windpipes suffered severe complications and subsequently died. His career and reputation unravelled, causing much soul-searching into the dangers of overhyping the progress of stem-cell therapy.
Questions of immunity
This case generated so much interest partly because it was perceived that patients could be treated with stem cells taken from their own bone marrow. Many such treatments have not worked, though the notable exception is blood stem-cell transplantation, which has been used on people with leukaemia and other cancers of the blood for decades, saving countless lives.
A recent report in the New Scientist detailed the treatment of diabetes in mice using genetically altered cells that bypass the immune system. Pioneered by Californian firm Sana Biotechnology, the method uses pancreas cells formed from stem cells that do not cause a destructive immune response. This is crucial because most stem-cell therapies in development require either the taking of immune suppressors or the use of stem cells derived from the person receiving them – which is costly, time-consuming and lacks universality. Cells from one person that are put into another usually provoke an immune response.
Sana Biotechnology bypassed this issue by genetically creating cells so they become invisible to the immune system. In tests using rhesus macaques, pluripotent versions of these cells – which have the potential to be turned into multiple different tissues and organs – survived with no sign of an immune attack for up to four months. By contrast, cells inserted into the monkeys without genetic changes were destroyed by their immune system within four weeks.
Blood tests indicated that the stem-cell-derived pancreas cells used to treat the mice for type 1 diabetes helped to reduce their diabetes symptoms. This research could be an important step towards off-the-shelf stem-cell treatment for a whole range of conditions including heart attacks and strokes. As Sana Biotechnology’s Sonja Schrepfer put it: “The vision is we have cells for anyone, anytime, anywhere.”
Future vision
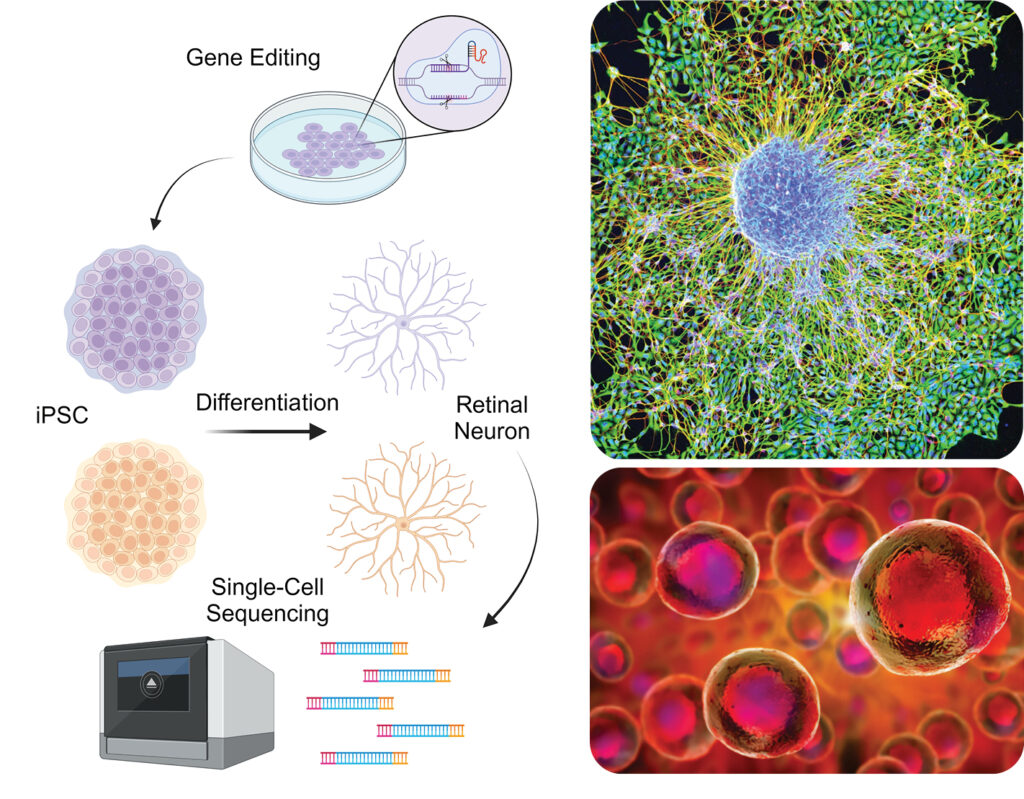
In a concerted effort to combat degeneration of the eye, Dr Chien-Ling Huang and her research team at the Hong Kong Polytechnic University’s Department of Health Technology and Informatics are utilising induced pluripotent stem cells (iPSCs), a unique manipulable cell type that can be obtained by reprogramming animal and human differentiated cells. The team not only harnessed the versatility of iPSCs but also refined the differentiation process, coaxing these cells into distinct lineages vital for blood circulation and the intricate structure of the retina.
Imitating nature, their approach involves crafting an artificial extracellular matrix, a scaffold providing structural and biochemical support for cell growth. They believe the innovation not only improves retinal neuron differentiation efficiency but also lays the groundwork for further advancements in regenerative medicine. “Through the strategic integration of state- of-the-art gene editing and advanced biomaterials, the team envisions a significant enhancement in the generation of specific cell types crucial for combating degenerative conditions,” said a spokesperson.
Dr Huang’s team indicated that they remain focused on the ultimate goal: to contribute significantly to the arsenal of medical interventions aimed at saving individuals from the grips of degeneration disorders.